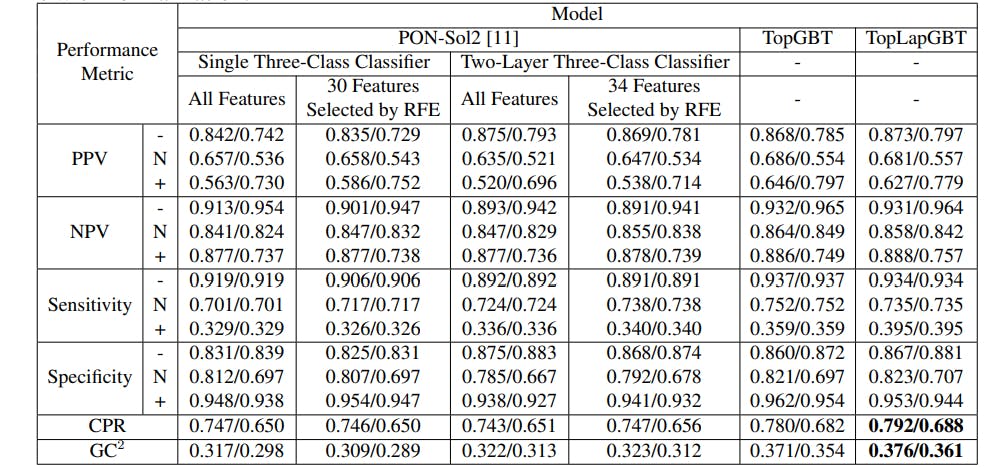
157 reads
TopLapGBT: A Unified Approach for Enhanced Protein Solubility Prediction
by
February 16th, 2024
Audio Presented by
Mutation: process of changing in form or nature. We publish the best academic journals & first hand accounts of Mutation
About Author
Mutation: process of changing in form or nature. We publish the best academic journals & first hand accounts of Mutation